Sterilizing solutions used in sterilization: Sterilizing pharmaceutical products and solutions inside the sterilization chamber prevents cross-contamination during the pharmaceutical output procedure.
his water is sterile WFI to which one or more acceptable antimicrobial preservatives happen to be added. This water is often intended for use being a diluent while in the preparing of sterile solutions, mainly for multi-dose items that require recurring information withdrawals, for example liquid pharmaceuticals. It could be packaged in solitary-dose or numerous-dose containers, commonly fewer than 30 mL.
. It may be used in other purposes which don't have particulate make any difference specifications, wherever bulk Water for Injection or Purified Water is indicated but exactly where use of a validated water procedure is not really practical, or where by rather more substantial quantities than are furnished as Sterile Water for Injection are needed. Sterile Water for Inhalation— Sterile Water for Inhalation (see USP monograph) is Water for Injection that is packaged and rendered sterile and is meant to be used in inhalators and while in the planning of inhalation alternatives.
Charge-Modified Filtration Charge-modified filters are generally microbially retentive filters which can be treated during their manufacture to have a constructive cost on their own surfaces. Microbial retentive filtration is going to be explained within a subsequent area, but the numerous aspect of these membranes is their electrostatic surface area charge. These kinds of billed filters can minimize endotoxin levels within the fluids passing via them by their adsorption (owing to endotoxin's adverse charge) on to the membrane surfaces. However ultrafilters tend to be more usually utilized to be a device Procedure for endotoxin removal in water techniques, cost-modified filters could even have an area in endotoxin removal specifically where out there upstream pressures are certainly not ample for ultrafiltration and for just one, comparatively short-term use. Demand-modified filters may be tricky to validate for lengthy-expression or big-quantity endotoxin retention.
Bacteriostatic WFI: This is certainly sterile Water for Injection that contains bacteriostatic (antimicrobial) brokers. It could be packed in one-dose containers of not larger sized than five ml dimension As well as in several-dose containers of not greater than 30 ml measurement, the label of which implies the identify as well as proportion of additional agent.
Microorganisms in biofilms depict a continual supply of contamination and are hard to right sample and quantify. For that reason, the planktonic population is normally used as an indicator of process contamination concentrations and is also The premise for program Warn and Action Degrees. The steady look of elevated planktonic concentrations will likely be an indication of State-of-the-art biofilm progress in need of remedial Handle. Program Regulate and sanitization are key in controlling biofilm formation plus the consequent planktonic populace.
These include things like process sensitivity, number of organisms types or species recovered, sample processing throughput, incubation time period, Value, and methodological complexity. An alternate thing to consider to using the classical “society” ways is a sophisticated instrumental or fast check technique that could yield far more timely benefits. Nonetheless, treatment must be exercised in picking such an alternative technique to make sure that it has equally sensitivity and correlation to classical society strategies, which happen to be usually viewed as the approved benchmarks for microbial enumeration.
employs elements that happen to be highly successful deionizers and that don't contribute copper ions or organics towards the water, assuring an exceptionally good quality water. If the water of the purity contacts the ambiance even briefly as it is actually staying used or drawn from its purification method, its conductivity will quickly degrade, by around about 1.0 µS/cm, as atmospheric carbon dioxide dissolves in the water and equilibrates to bicarbonate ions. Thus, When the analytical use calls for that water purity stays as high as you possibly can, its use ought to be protected against atmospheric exposure. This water is used as a reagent, for a solvent for reagent planning, and for take a look at equipment cleaning where fewer pure waters wouldn't execute acceptably. However, if a user's routinely available purified water is filtered and satisfies or exceeds the conductivity specifications of Superior Purity Water, it may be used in lieu of Large Purity Water. Ammonia-Totally free Water— Functionally, this water need to have a negligible ammonia concentration to stop interference in tests delicate to ammonia. It's been equated with Significant Purity Water that features a appreciably tighter Stage one conductivity specification than Purified Water due to the latter's allowance for any nominal volume of ammonium between other ions. On the other hand, When the consumer's Purified Water were filtered and fulfilled or exceeded the conductivity technical specs of Substantial Purity Water, it might contain negligible ammonia or other ions and will be used in lieu of Large Purity Water. Carbon Dioxide-Absolutely free Water— The introductory percentage of the Reagents, Indicators, and Remedies segment defines this water as Purified Water that's been vigorously boiled for at least 5 minutes, then cooled and shielded from absorption of atmospheric carbon dioxide. As the absorption of carbon dioxide has a more info tendency to push down the water pH, the vast majority of employs of Carbon Dioxide-Free Water are both associated to be a solvent in pH-related or pH-delicate determinations or to be a solvent in carbonate-delicate reagents or determinations. A further use of this water is for sure optical rotation and shade and clarity of Alternative exams. Though it is possible that this water is indicated for these tests simply because of its purity, it is also doable that the pH effects of carbon dioxide that contains water could interfere with the outcome of these assessments. A 3rd get more info plausible explanation that this water is indicated is always that outgassing air bubbles might interfere Using these photometric-type checks. The boiled water preparing approach will even tremendously lowered the concentrations of a number of other dissolved gases in addition to carbon dioxide. For that reason, in many of the apps for Carbon Dioxide-Free of charge Water, it could be the inadvertent deaeration impact that truly renders this water suitable.
Seasonal versions in temperature and progress of flora may additionally result in fluctuations in microbial written content of resource water. Checking need to be Regular more than enough to deal with these variations.
Microbial-Retentive Filtration Microbial-retentive membrane filters have professional an evolution of knowing in the past decade which has caused Formerly held theoretical retention mechanisms to be reconsidered. These filters have a bigger helpful “pore sizing” than ultrafilters and are meant to avoid the passage of microorganisms and similarly sized particles without having unduly restricting circulation. This type of filtration is greatly utilized within water devices for filtering the germs out of both water and compressed gases along with for vent filters on tanks and stills and various unit functions. Nonetheless, the Houses of your water method microorganisms seem to obstacle a filter's microbial retention from water with phenomena absent from other aseptic filtration apps, like filter sterilizing of pharmaceutical formulations previous to packaging. Within the latter software, sterilizing grade filters are commonly viewed as to own an assigned score of 0.
The latter four waters are "finished" items that are packaged and labeled as a result and want not be of concern in the course of an inspection outside of vegetation which basically develop these goods.
In watch of possible apps on mobile-based immunotherapy, the objective of this review was to validate the influence of advancement in 3D spheroids generated on hugely hydrorepellent surfaces over the morphology, viability, and polarization of derived cell entities. With this work, the preparation and physicochemical characterization of remarkably water-repellent surfaces to build and characterize 3D spheroids derived from monocyte-macrophages (Uncooked 264.
Purified Water should meet up with the requirements for ionic and organic and natural chemical purity and need to be shielded from microbial contamination.
Validation is the process whereby substantiation to a high volume of assurance that a specific system will continually generate an item conforming to a longtime set of top quality characteristics is acquired and documented. Before and during the quite early stages of validation, the significant method parameters as well as their functioning ranges are set up.
 Val Kilmer Then & Now!
Val Kilmer Then & Now!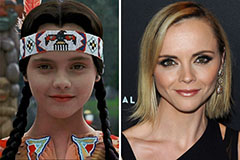 Christina Ricci Then & Now!
Christina Ricci Then & Now! Tyra Banks Then & Now!
Tyra Banks Then & Now! Andrew McCarthy Then & Now!
Andrew McCarthy Then & Now! Ryan Phillippe Then & Now!
Ryan Phillippe Then & Now!